Sometimes things change. When a toast burns, it is no longer the same substance. Its molecules have been rearranged by heat. What is left is carbon, an entirely new substance. This is called a chemical change. However, when ice turns to water and then to a gas, the molecules of water do not change. The forms which the substance takes change but the substance, water, does not. This is called a natural change. Let’s see how this works.
Things Required:
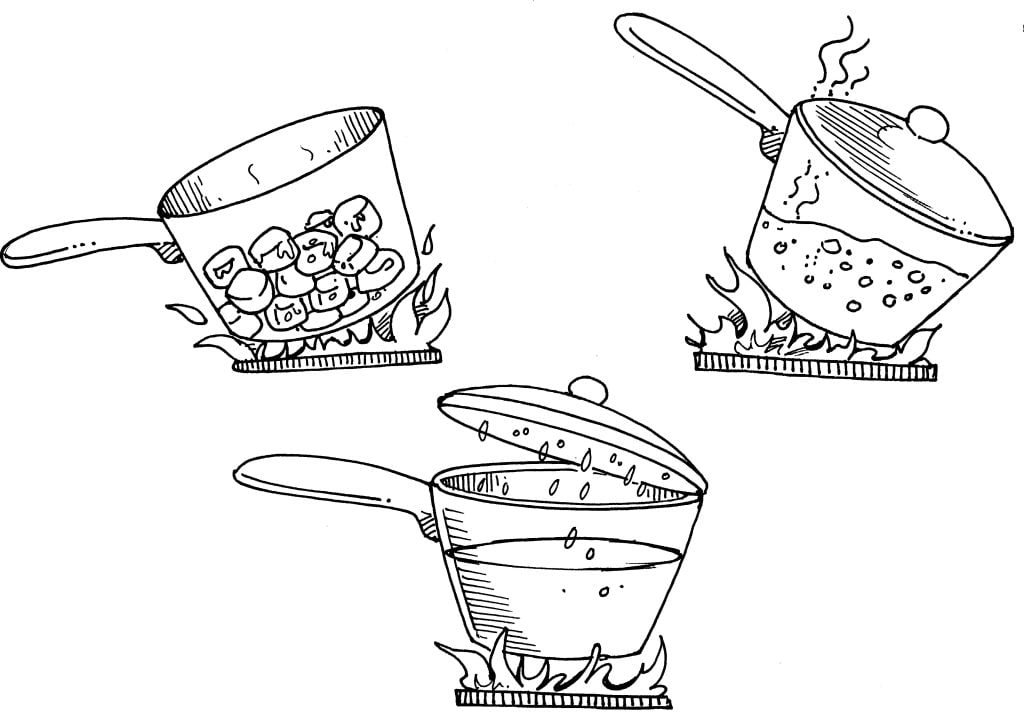
10 ice cubes
A small pot with lid
Use of stove
Directions:
Put the ice cubes in the pot and melt them on the stove. Once the cubes turn to water and the water starts to boil, place the lid on the pot. Let it boil for a few minutes, and then turn the heat off and let the pot cool. Then, as you lift the lid, observe the water drops on boiling water the underside.
This Is What Happens:
The ice turns to water, the water to steam, a gas which we sometimes call water vapour, and the steam back into water.
Science Behind It:
Ice is a solid. Its molecules move slowly but they do move. When you heat the ice cubes, the molecules move faster. The ice gets warmer and melts. When you continue to heat the water further, the molecules move even faster, bump into one another, and escape, leaving the liquid. The water drops that collect on the inside of the lid are a result of water vapour (gas). As the pot cools, the vapour turns back into water (liquid). This process is known as condensation. Chemists identify what happens in this experiment as demonstrating the three states of matter: solid, liquid and gas.

