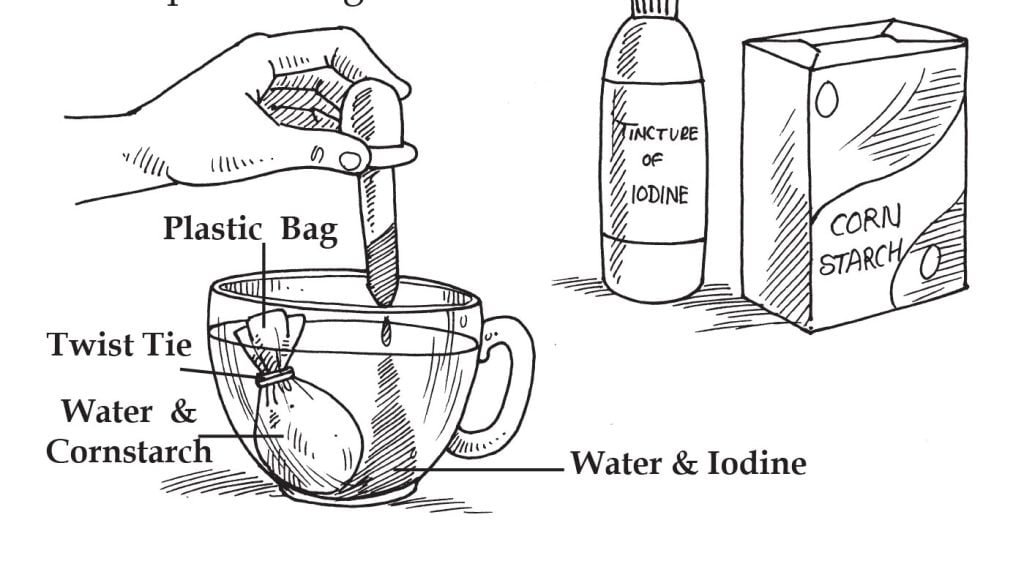
The function of this experiment is to observe the movement of particles through a membrane.
Things Required:
1 plastic sandwich bag
1 twist tie
Tincture of iodine
Cornstarch
2 measuring cups (500 ml)
Eye-dropper
Measuring spoon -tablespoon (15 ml)
Directions:
Fill a cup one-half full with water, and add 20 drops of iodine. Fill a second cup with water and stir in one spoon of cornstarch. Pour one-half of the starch and water mixture into the plastic bag. Use the twist tie to secure the top of the bag.
Rinse off any starch and water mixture that might have dropped onto the outside of the bag. Place the bag in the cup of water and iodine. Observe any changes immediately and then after 30 minutes.
While waiting for changes to occur inside the bag add 5 drops of iodine to the remaining starch and water mixture in the cup.
This Is What Happens:
The iodine turns the starch and water mixture in the cup black. The iodine does not turn the water in the bowl black, but after a while the contents of the plastic bag turn black.
Science Behind It:
Iodine is used to test for the presence of starch since a purple-black colour forms when the two materials are mixed. The water in the bowl never turned black indicating the absence of starch in the water. The iodine particles are small enough to move through the tiny holes in the plastic bag, but the starch molecules are too large to pass through. The inside of the bag turned black because the iodine passed through the membrane and mixed with the starch inside. The water outside contains iodine, but the starch was unable to move out of the bag, thus no colour change.
The plastic bag represents a cell membrane with cell parts inside. Materials are able to move into and out of the cell through this membrane. This movement of materials through a membrane is called osmosis.
q q

