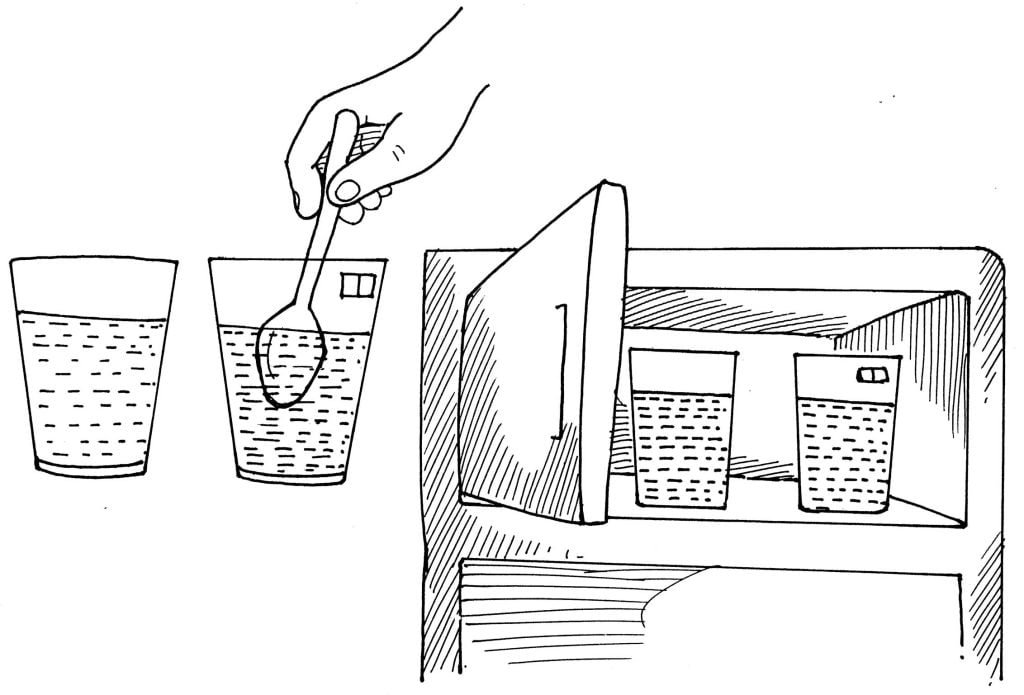
The purpose of all the effort is to show that salt makes it harder for water to freeze.
Things Required:
Two 5-oz paper cups
1 tablespoonful of table salt
Marking pen
Directions:
Fill both cups one-half full with water. Dissolve 1 tablespoonful of salt into one of the cups. Mark an S on the cup containing the salt. Set both cups in the freezer of a refrigerator. Check the cups every 30 minutes for one day, then leave the cups for 24 hours.
This Is What Happens:
The salty water never freezes.
Science Behind It:
The salt causes the water to freeze at a lower temperature. At the freezing temperature of water, 0°C, the water molecules start linking together to form ice crystals. The salt gets in the way of this linking process, and a lower temperature is needed before the water can freeze.

