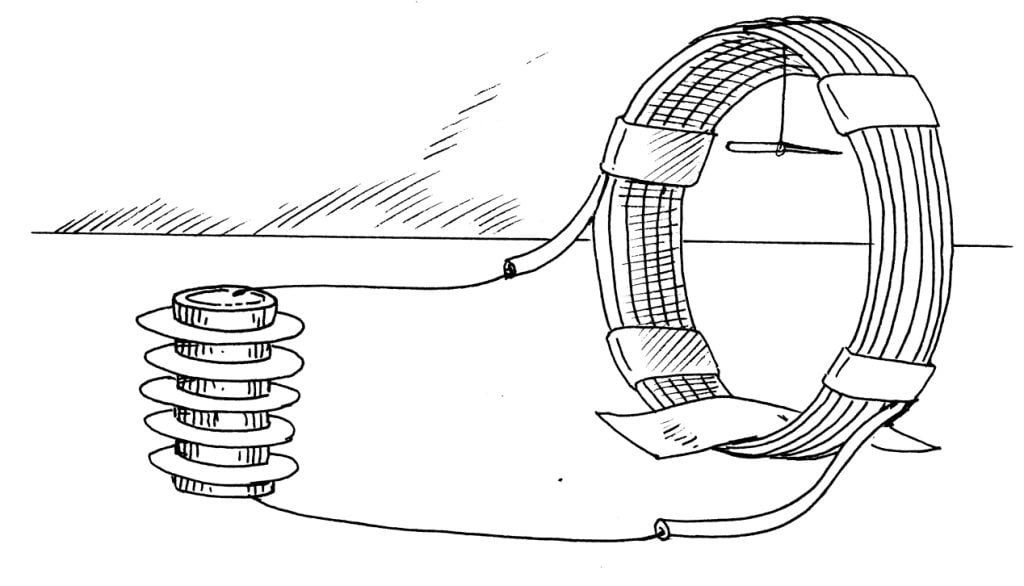
A single cell made of two metals can produce only a small “push” of charge. To increase this push, several cells can be wired together. This arrangement of side-by-side cells forms an electrical device known to scientists as a battery.
Things Required:
Three copper coins
Three iron washers
Steel wool
Blotter paper
Pair of scissors
Salt
Water
Current meter (assembled in “Simple Multimeter”)
Directions:
Polish the surface of the coins and washers with steel wool. Use a pair of scissors to cut the blotter paper into four coin-sized circles.
Soak the paper in salt water. Sandwich a damp paper circle between a coin and a washer. Test the generation of electricity with your current meter. How does the meter react?
Make two other sandwiches. Stack all three sandwiches into a pile of alternating coin/wet paper/washer units. Insert a saltwater-soaked paper between the coin of one cell and the washer of its neighbour. In other words, no two coins should make direct contact.
Use tape to secure this three-cell stack. Retest the current. Is it stronger? Can you explain your observations?
This Is What Happens:
The simple coin/washer cell produced a very small amount of electricity. As the cells were joined together, they formed a battery. The three-cell battery generated three times the electricity of a single cell. This increase produced a more noticeable deflection of the needle of the meter.

