Hot cereal feels good, especially on a cold morning. Does it matter whether you start it in cold or boiling water?
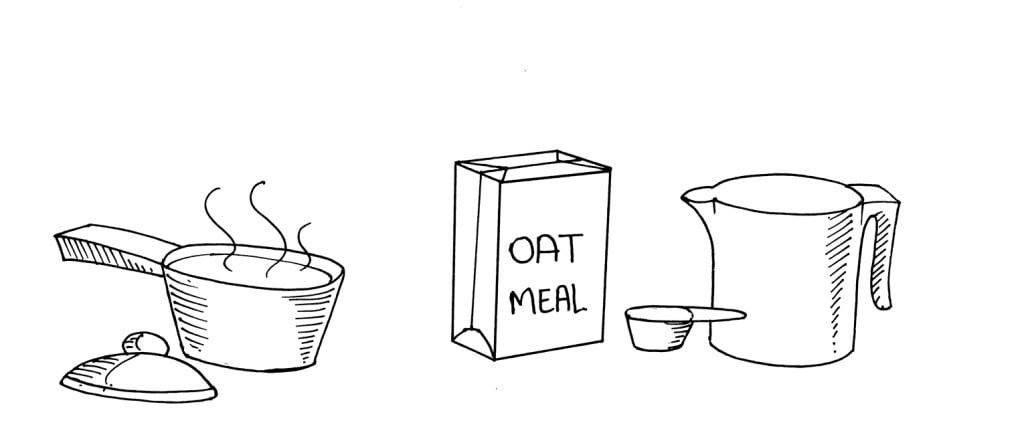
Things Required:
1½ cup of water
2/3 cup of oatmeal
2 small pots
Salt (optional)
Directions:
Stir 1/3 cup of the oatmeal into a pot with ¾ cup of the water. Bring it to a boil, lower the flame, and simmer for five minutes, stirring occasionally. Cover the pot and remove it from the heat. Let the mixture stand.
In a second pot, bring the rest of the water to a boil. Add salt and pour to the other half of the oatmeal. Lower the flame and simmer for five minutes, stirring occasionally. Again, remove the cover from the heat and let the mixture stand for a few minutes.
Taste the first pot of oatmeal. Then taste the second one.
This Is What Happens:
Both are cooked and taste good. The oatmeal that started in the cold water is creamier than the oatmeal that started in boiling water.
Science Behind It:
As you heat the grains, the starch granules absorb water molecules, swell and soften. Then the nutrients inside are released and are more easily absorbed by the body.
When you start cooking the oatmeal in cold water, the granules have a longer time to absorb the water. The activity starts at 140°F (60°C), well below the 212°F (100°C) boiling point. The complex carbohydrates (amylose and amylopectin) that make up the starch change. They break up some of the bonds between the atoms of the same molecule and form new bonds between the atoms of different molecules. The water molecules then get trapped in the starch granules, which become bulky and eventually break, releasing the nutrients inside.

