Since salt and flour were so good in “All Mixed Up!” let’s bring them back. So, here they are again, friends – salt and flour!
Things Required:
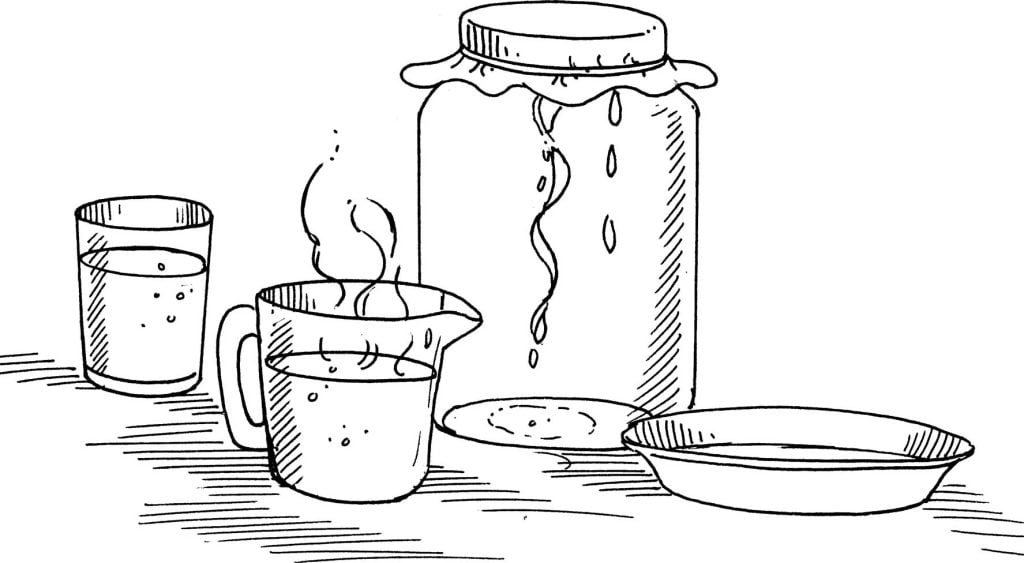
A wide-mouth jar
Coffee filter
Salt and flour mixture (from the last experiment)
Hot tap water
A shallow container
A rubber band
Directions:
Place the filter over the top of the jar and put the rubber band around it to keep it in place. Let the filter droop or sag a little in the middle so that it may hold the water more easily. Pour the salty water and flour mixture from the last experiment slowly onto the filter. Very slowly, add a little bit of hot water to help the salt solution break through the flour. Be patient! It will take some time to recover or get back a good amount of salt solution. Save as much as you want, and then take the filter off the jar. Pour the salt solution out of the jar into the shallow container. Let it stand in a warm place for 24 hours.
This Is What Happens:
The flour stays on the top of the filter while the salt in the water passes through it. When the water eventually evaporates from the shallow container, it leaves salt crystals behind.
Science Behind It:
The molecules of solid salt crystals (called a solute by chemists) that had dissolved in the water (solvent) could pass freely through the filter, while the flour grains, which are much too large and do not dissolve, remained on top.
Because water evaporates but salt cannot, the salt molecules left behind as the water disappeared reformed into crystals.

