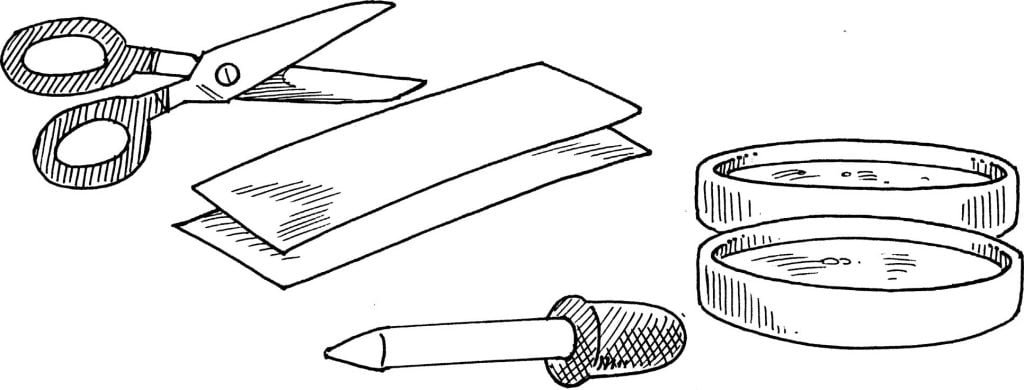
Things Required:
2 small shallow containers
2 tablespoonfuls of oil
2 tablespoonfuls of water
White construction paper
Scissors
Paper towels or napkins
Food colouring
A medicine dropper
Directions:
Place the oil into one shallow container and the water into the other. Cut two small strips from the construction paper. Dip one paper strip into the oil and the other into the water; then place them on the paper towels or napkins. Drop a drop of food colouring on each.
This Is What Happens:
The drop of food colouring on the oiled paper sits on the surface while the drop on the water-dipped paper spreads out.
Science Behind It:
The food colouring, which is water-based, sits as a drop on the oiled paper because its water molecules will not combine with the oil. A substance is called “immiscible” with another when it does not combine to become one substance. The food colouring on the water-dipped paper is said to be “miscible” with it. It dissolves on the paper strip and spreads out, even beyond the paper. Its molecules combine as do the molecules in a solution.

