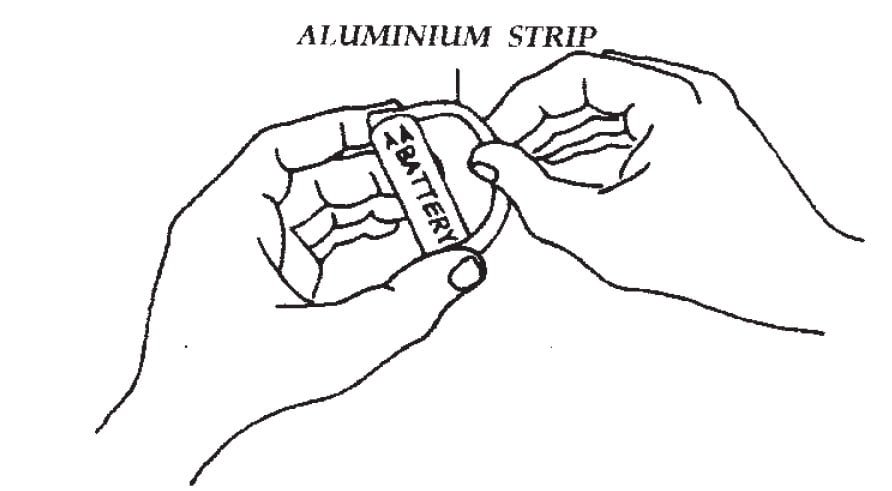
The purpose of this exciting experiment is to discover that the flow of electrons generates heat.
Things Required:
1 AA battery
Aluminium foil
Scissors
Ruler
Directions:
Cut a strip from the aluminium foil, 6 inches × 1 in. (15 cm × 2.5 cm). Fold the strip of foil in half lengthwise twice to form a thin 6-inches (15-cm) strip that will be used as a wire. Using one hand, hold one end of the aluminium wire against each battery pole. After 10 seconds, touch the aluminium wire while you continue to hold the wire against the battery ends.
Caution: Do not hold the wire against the battery ends longer than 20 seconds. The wire will continue to get hot and the battery is being discharged (losing its power).
This Is What Happens:
The aluminium wire gets hot.
Science Behind It:
Touching the wire to the ends of the battery produces a path through which electrons travel (an electric circuit). The electrons move out of the negative end of the battery through the wire and back into the positive end of the battery. The movement of the electrons causes the wire to get hot.
When a light bulb is placed in the electrical circuit, the electrons move through the bulb. The movement of the electrons heats up the wire filament inside the bulb. The hot wire filament becomes incandescent, that is, it gets so hot that it gives off light.

