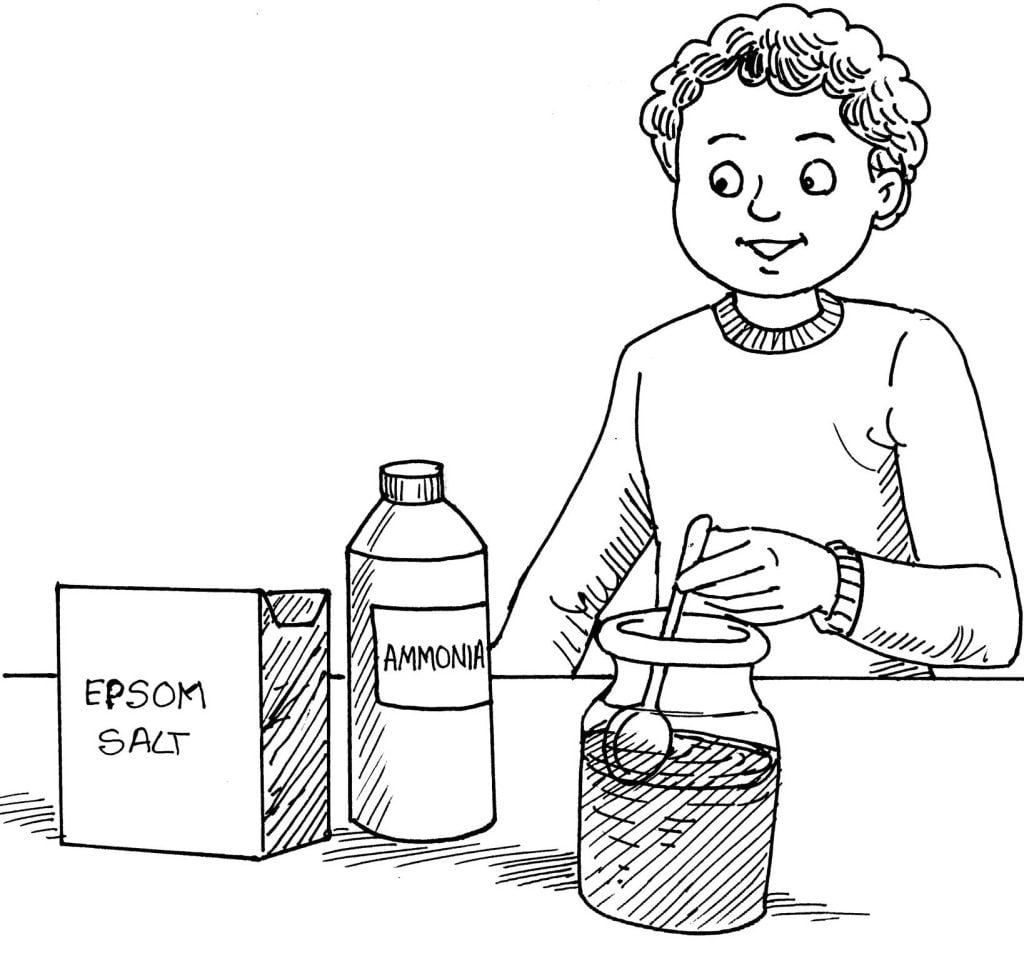
Here, you will learn how to make a milky, magnesia solution.
Things Required:
1 teaspoonful of epsom salt
2 teaspoonfuls of household ammonia
1 small baby food jar
Directions:
Fill the jar one-half full with water. Stir 1 teaspoonful of epsom salt into the water. Pour 2 teaspoonfuls of ammonia into the jar. Do not stir. Allow the solution to stand for five minutes.
This Is What Happens:
A white, milky substance forms as the ammonia mixes with the epsom salt solution.
Science Behind It:
The chemical name of household ammonia is ammonium hydroxide. Magnesium sulphate is the chemical name for epsom salt. Mixing ammonia and epsom salt causes a reaction which produces magnesium hydroxide as one of the products. Magnesium hydroxide is a white substance that does not dissolve well in water. After standing a while, the white floating particles settle to the bottom of the jar. Magnesium hydroxide is part of the medicine called Milk of Magnesia. The name “milk” is used because of the milky appearance.
