
An easy way to start learning about atoms is to make a model of an atomic orbit. Although electrons and protons are not clay balls (in fact, electrons are fast-moving; electrically charged particles that move faster than you can say “atom”). Making a clay model will help you understand that it can be a very difficult idea.
Things Required:
4 colours of modelling clay
Wide-mouth jar lid
Newspaper (to cover work area)
Directions:
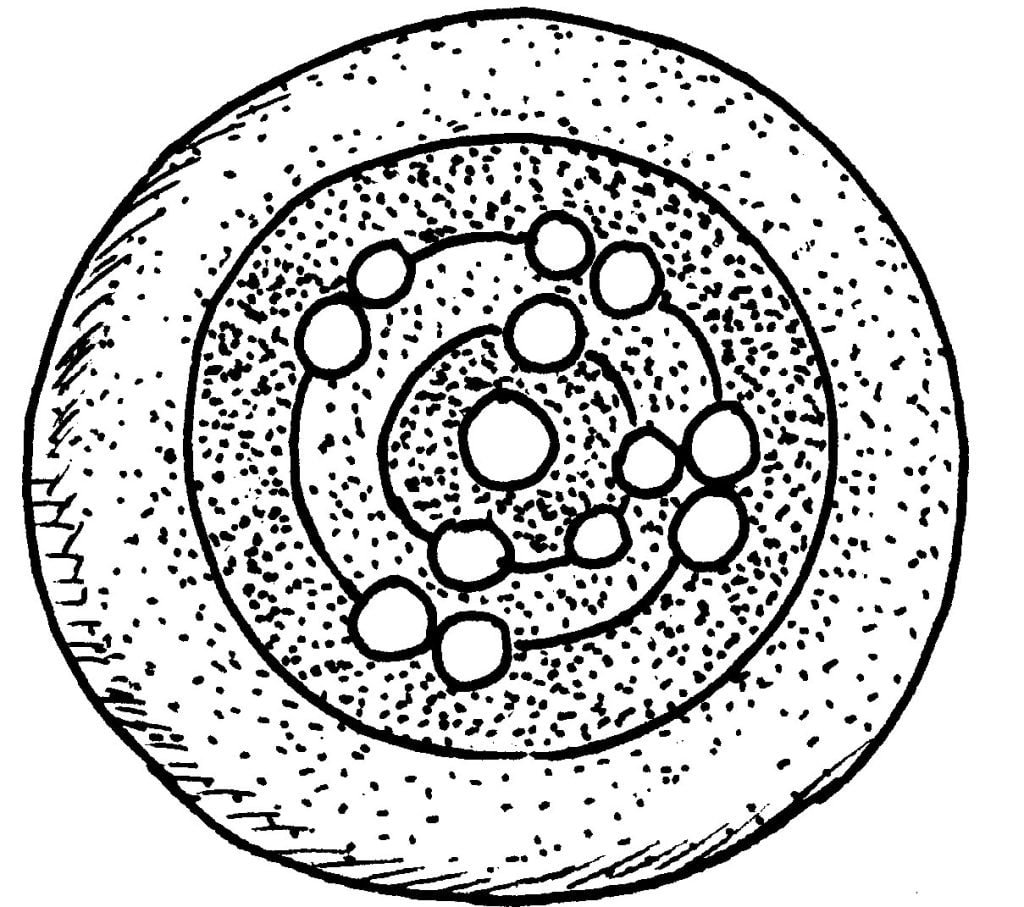
Spread some newspaper over your work area. Select any two colours of clay. We’ll use red and blue. Now, make two red clay ropes and a blue rope, rolling them out with your hands. These will show the orbits or shells or paths the electrons will take around the nucleus. Make certain that you make the ropes long enough to make complete circles inside the jar lid. Press the first red rope against the inner rim of the lid. Follow it with the blue rope, pressed in next to the red. (When you have finished your model, it will have a target pattern.) Now press another circle of red in next to the blue rope; then place a blue “bull’s-eye” piece of clay in the middle. When you have finished, flatten the clay with your fingers.
Next, make a “yellow” clay ball and stick it on the “bull’s-eye”. Make two smaller (green) balls and stick these to the outside of the blue bull’s-eye, one on each side and in a straight line with the larger ball. Then place eight more green balls in four groups of two on the outer edge of the red ring.
This Is What Happens:
You now have made a usable atomic model!
Science Behind It:
Atoms can have no more than seven orbits, or paths, and only so many electrons can fit into each orbit. The larger ball in the “bull’s-eye” represents the nucleus of the atom. The two smaller balls on the outer edge of the blue circle show that there are only two electrons in the first orbit. The second orbit, the edge of the red ring, has eight green balls around it, showing that only eight electrons can be in its orbit.
The third orbit of the model (outer edge of the blue clay circle, not filled) can have up to eight more green balls, or electrons, if it is the last orbit, but up to eighteen, if it is not the last. An important thing to remember is that, after the first orbit, each orbit in turn must have eight electrons before another orbit is started.

