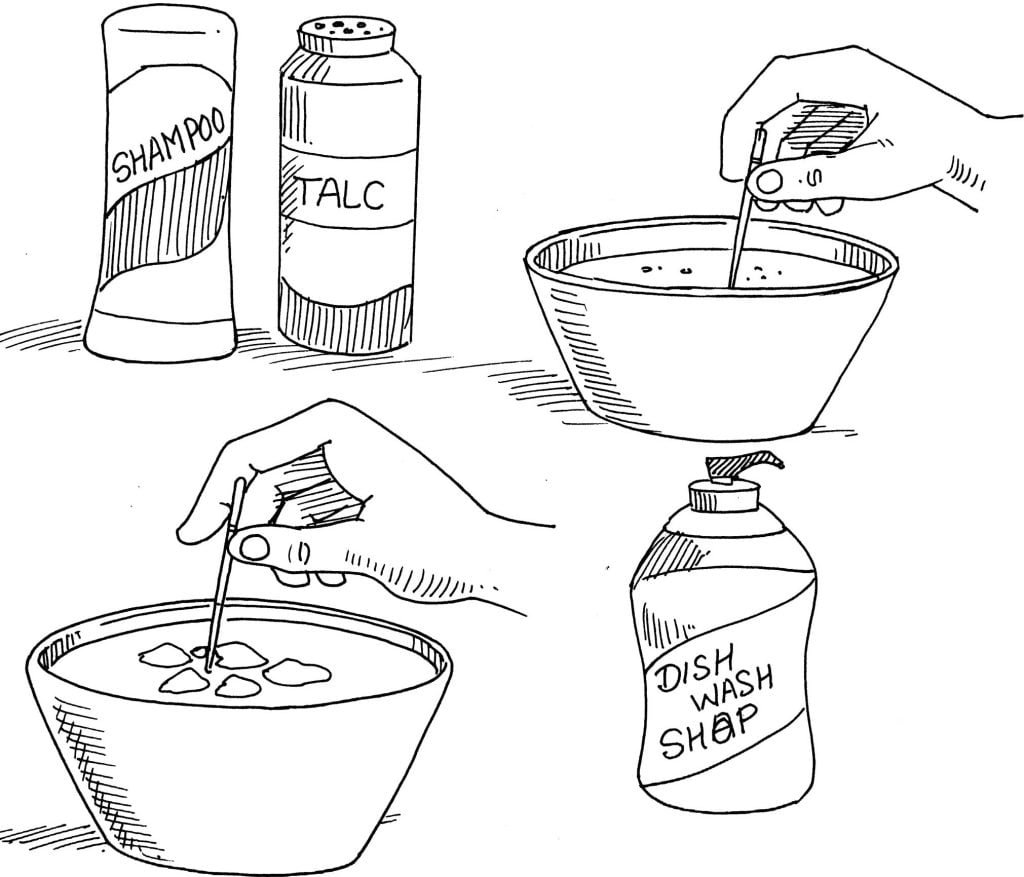
Best experiment to understand the wetting effect of soap and detergent
Things Required:
Shampoo
Liquid dish soap
Toothpicks
Talcum powder
2 soup bowls
Directions:
Fill both bowls with water. Sprinkle a thin layer of talcum powder on the surface of the water in each bowl. Dip the end of one toothpick in the shampoo, and touch the end in the centre of the powder in one bowl. Observe the movement of the powder.
Dip the end of a second toothpick in the liquid dish soap and touch the end in the centre of the powder in the second bowl. Observe the movement of the powder.
This Is What Happens:
Shampoo makes the talcum powder break like large floating ice blocks. The powder rushes to the sides of the bowl and starts to sink when touched by liquid dish soap.
Science Behind It:
Talcum powder is water-resistant. The grains of powder float on top of the water. The water molecules on the surface are pulling equally in all directions before the shampoo or dish soap is added. The addition of the shampoo or dish soap breaks the attraction between the water molecules wherever it touches, causing the water to move outward and taking the floating powder with it. The shampoo is a pure soap while the liquid dish soap is actually a detergent. Detergents are wetting agents and soap is not. A wetting agent is able to spread rapidly over the surface of a solid and penetrate the surface. The liquid dish soap dissolves in the water and quickly covers the grains of talcum powder, causing them to sink to the bottom of the bowl.

