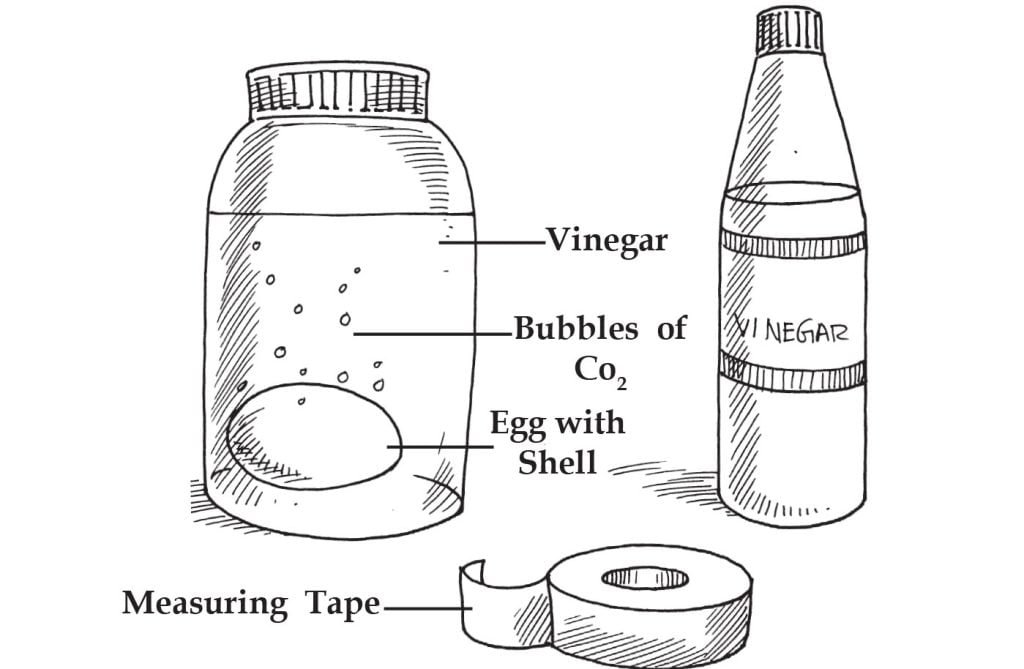
In this experiment, we will observe the semi-permeability of a cell membrane.
Things Required:
1 raw egg in its shell
1 jar with a lid (the egg must fit inside the jar)
Clear vinegar
Flexible measuring tape
Directions:
Measure and record the circumference around the centre of the egg. Record the appearance of the egg. Place the egg inside the jar. Do not crack the shell. Cover the egg with vinegar.
Close the lid. Observe immediately and then periodically for the next 72 hours.
Remove the egg after 72 hours and measure its circumference. Compare the appearance of the egg before and after being in the vinegar. How has the egg changed in appearance and size? Keep the egg for the Shrinking Egg Experiment.
This Is What Happens:
The egg has a hard shell on the outside and the circumference will vary. Bubbles start forming on the surface of the shell of the egg immediately and increase in number with time. After 72 hours, the shell will be gone and portions of it may be seen floating on the surface of the vine-gar. The egg remains intact because of the thin see-through membrane. The size of the egg has increased.
The shell of the egg is made of calcium carbonate, commonly called limestone. When vinegar chemically reacts with the limestone, one of the products is carbon dioxide, those bubbles seen on the egg. The membrane around egg does not dissolve in vinegar, but becomes more rubbery. The increased size is due to osmosis, the movement of water through a cell membrane. The water in the vinegar moves through the thin membrane into the egg because the water inside the egg has more materials dissolved in it than does the vinegar. Water will always move through a membrane in the direction where there are more dissolved materials. The contents of the egg stayed inside the membrane because these molecules were too large to pass through the tiny holes. This selectiveness of materials moving through the membrane is called semi-permeability.

