How would you like to make your own bath solution? This solution will make your water softer, and soft water makes more suds to clean you better. It’s fun and easy, and it’s real chemistry.
Things Required:
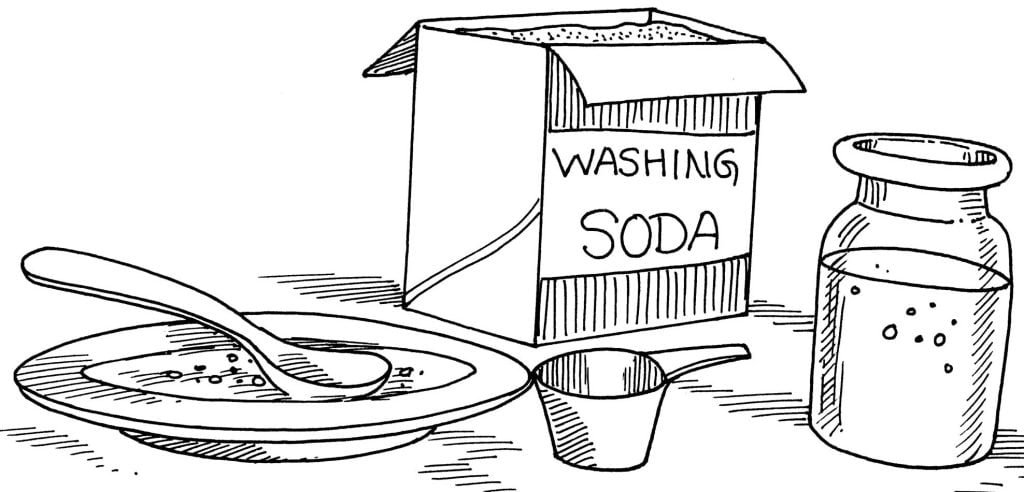
1/2 cup of washing soda
A clean, medium-size jar with lid, filled with water
A saucer or small dish
A spoon
Directions:
Pour some of the washing soda into the saucer. With the back of the spoon, crush the crystals of washing soda into a fine powder. Spoon the washing soda powder into the jar of water a little at a time until no more will dissolve. (If needed, crush more washing soda powder and add it to the jar of water.) You now have a “saturated solution”. Store the solution in the jar and add a small amount to the water when you take your bath.
This Is What Happens:
The washing soda dissolves in the water and creates a natural bathwater softener.
Science Behind It:
Sodium carbonate or washing soda neutralizes or softens water by removing hard bath salts, such as calcium. When this happens, chemists call it precipitation, but unlike weather forecasters, they don’t mean rain the soft water that falls from the sky. Here, precipitation is the chemical splitting of the two compounds, calcium salts and washing soda, into simpler molecules which form a solid substance, called a precipitate.

